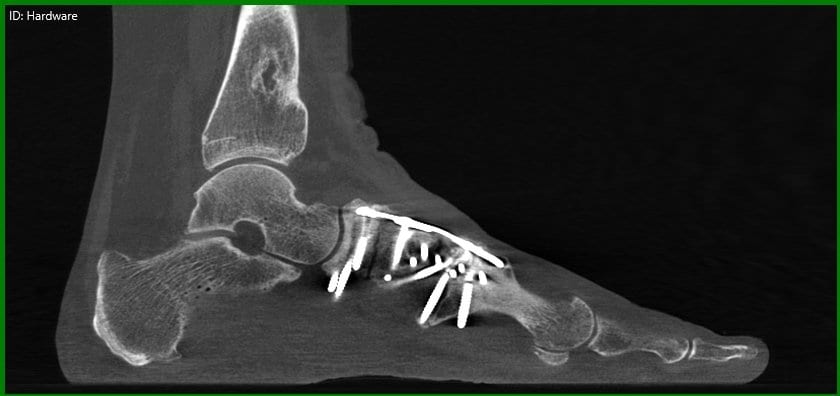
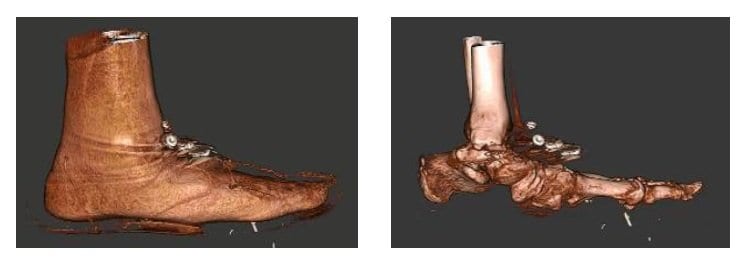
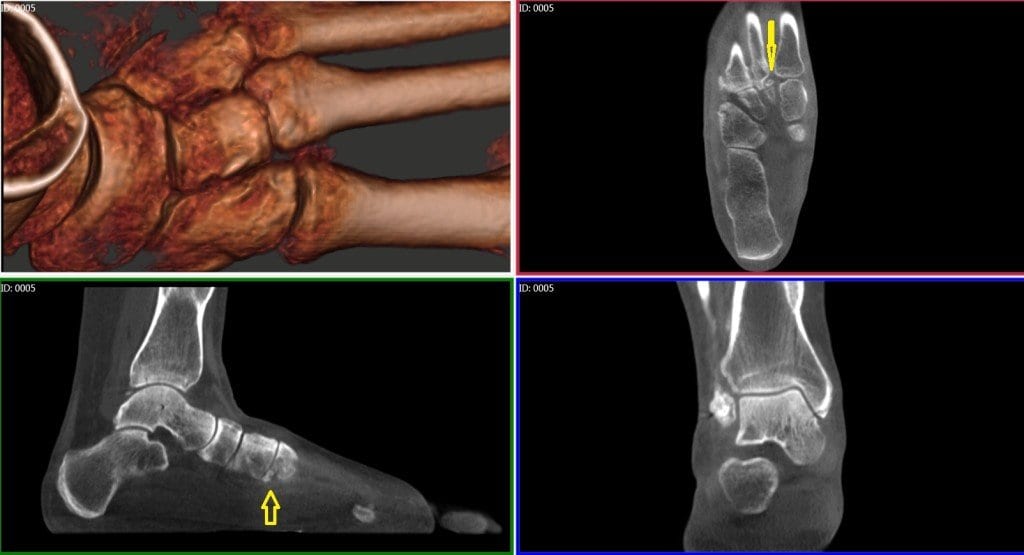
X-rays of the foot and ankle may not always provide conclusive assessments for post-operative fusions such as of the tarso-metatarsal joint or hind foot joints. Similarly, the physician is often left guessing if a fracture has properly healed.
“The pedCAT takes all the variability out, all the guesswork out of it,” Dr. Martin O’Malley, MD, an Associate Attending Orthopedic Surgeon at the Hospital for Special Surgery in New York, New York, said.
Dr. O’Malley said pedCAT scans allow him to clearly determine if a fusion has healed more than 50 percent, and he decides when to ambulate his patient accordingly.
“I let people walk on it earlier than before, and I keep them off longer than before,” Dr. O’Malley said.
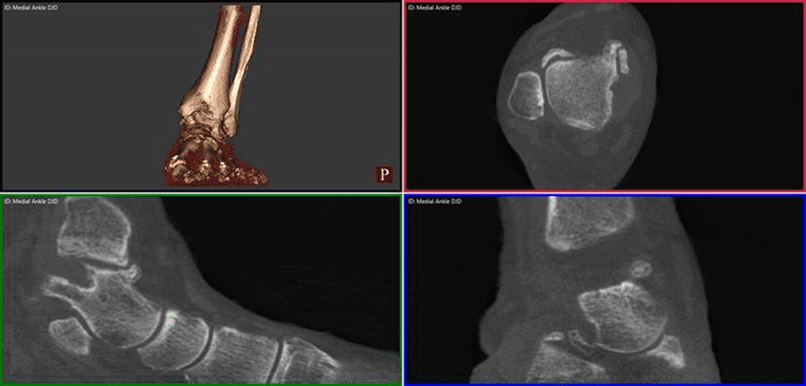
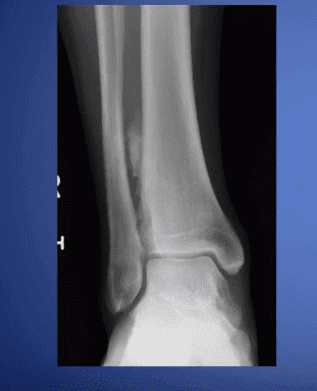
The pedCAT provides a three-dimensional view of fractures that changes the way O’Malley sees this common diagnosis.
“These posterior pieces are often bigger than we thought were based on plain X-ray and they often travel all the way around the medial side as well, which we never thought they did,” Dr. O’Malley said. “You know, we thought it was an infrequent fracture, but now we see it routinely. Now most of my ankle fracture work, I’d say more than half the time, is through a posterior approach. For the first 15 to 18 years of my practice I would do medial/ lateral incisions. Now I’m going to the back of the ankle. And a lot of it is driven by the pedCAT.”
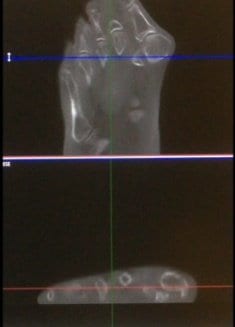
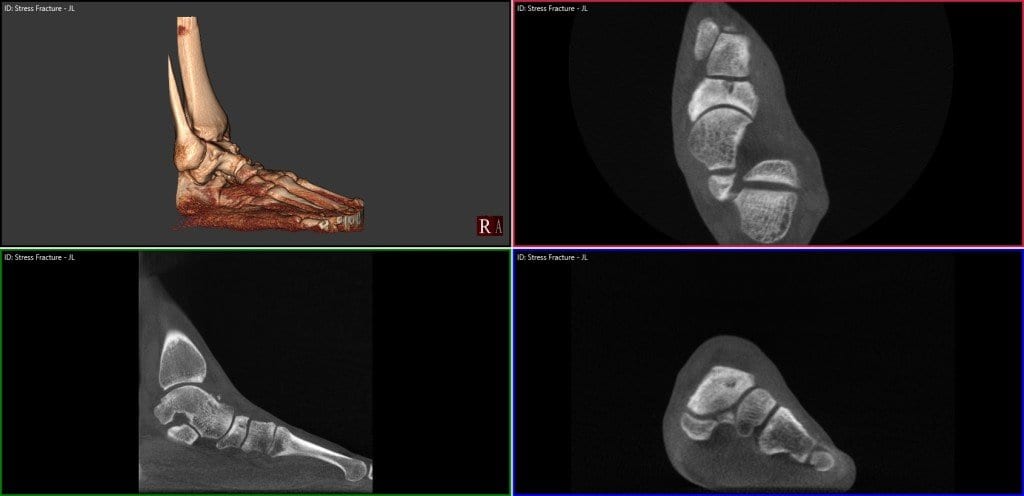
pedCAT Stress Fracture Cross Section
Click on the blog post title to see an example of a navicular stress fracture in a collegiate runner that had not healed at all after six weeks of casting. Were it not for the conclusive pedCAT scan, this patient would have been allowed to ambulate.
Watch Dr. O’Malley talk more about the pedCAT here .
To offer your patients state-of-the-art fracture & fusion assessment, consider a pedCAT for your practice.